THE DISEASE - MALARIA
WHAT IS MALARIA?
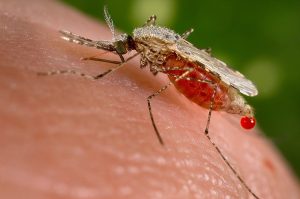
Malaria is a life-threatening disease caused by parasites transmitted through the bite of infected mosquitoes.
Pregnant women are particularly vulnerable to malaria and should take extra precautions to avoid mosquito bites and follow antimalarial medication guidelines under medical supervision.
Because a century of work on control and eradication has failed to eliminate this scourge.
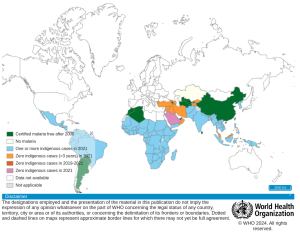
The Fight against malaria
Malaria can lead to various complications, including anemia, neurological damage, and organ failure, which may have long-term effects on health if not treated promptly.
CURRENT VACCINE STRATEGIES
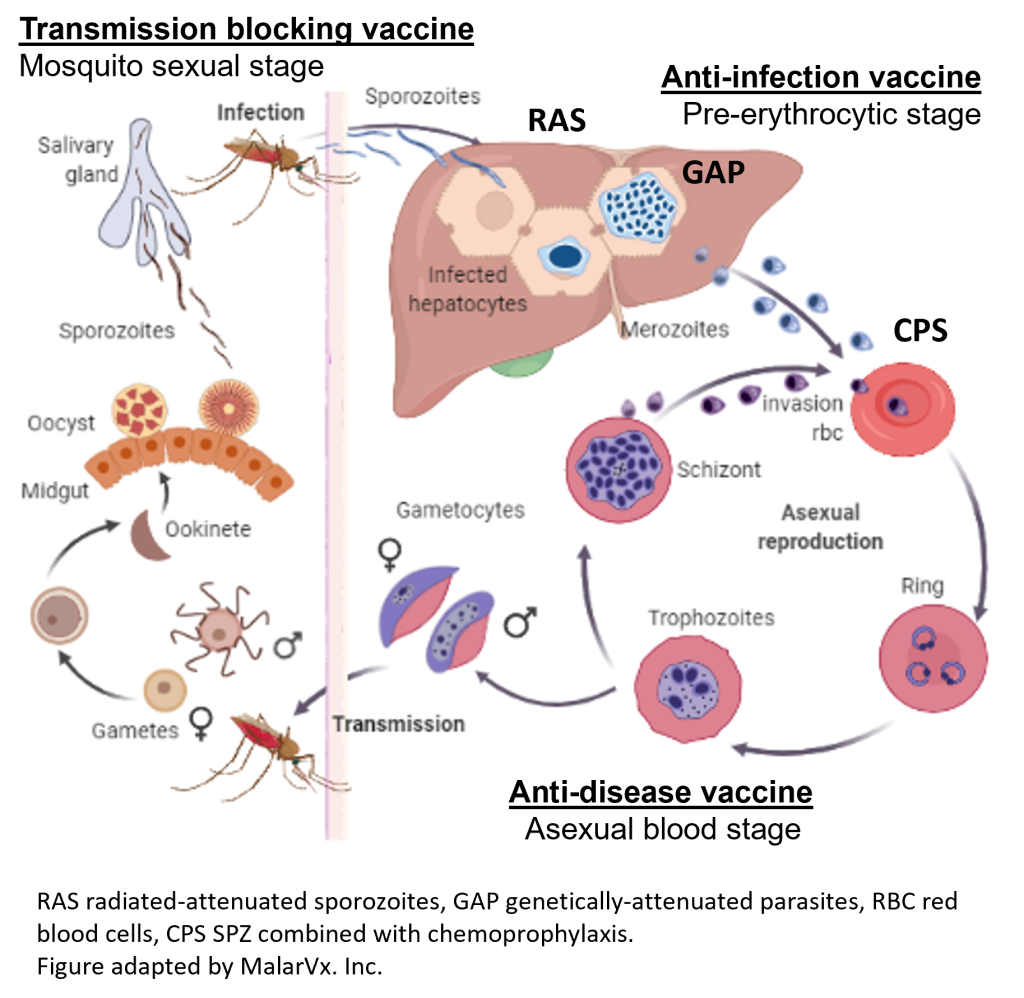
Multiple vaccines strategies can offer progress in the fight against malaria, particularly in high-burden regions, however these vaccines require several clinic visits and modest efficacy.
Continued research and development are essential to improve efficacy, extend protection duration, and develop new vaccines targeting different malaria strains.
Interrupting infection during the mosquito-to-skin-to-liver (“pre-erythrocytic”, PE) stages would prevent progression to the erythrocytic stage, where both disease and transmission occur (Draper et al. 2018; Kurtovic et al. 2021).
RAS: “Sterile protection”
GAP: Genetically-attenuated parasites (GAP) that arrest in the liver have been generated by deleting genes critical for liver-stage development (Vaughan et al. 2018; Kublin et al. 2017; Butler et al. 2011). Newer GAPs progress further into the liver stage, where they provide improved protection at lower doses (Goswami et al. 2020; Goswami, Minkah, and Kappe 2019).
CPS: Likewise, immunization with wild-type (WT) WO SPZ combined with chemoprophylaxis (CPS) can induce high levels of protection with smaller doses (Mwakingwe-Omari et al. 2021). In these WO approaches, primed T cells from the spleen are drawn to the liver, where antigen presentation is essential to generate protective liver-resident CD8+ T-cell memory responses (Krzych et al. 2012).
This type of vaccine aims to halt the spread of malaria by interrupting the life cycle of the parasite, thus reducing transmission within the population. It induces an immune response that inhibits parasite development in the mosquito.
As of 2024, there are two notable Malaria vaccines that have been recommended by the WHO for use, but those lack sterile protection:
Approval: Approved by the World Health Organization (WHO) in October 2021 for broader use.
Target Population: Designed for children aged 6 weeks to 17 months. Efficacy: Demonstrated to reduce malaria cases by about 39% and severe malaria by 29% over four years of follow-up in young children.
Implementation: It has been rolled out in pilot programs in several African countries including Ghana, Kenya, and Malawi. The WHO recommended its broader use in 2021 after observing its effectiveness and safety in these pilots.
Approval: Approved for use in Ghana and Nigeria as of April 2023. Target Population: Primarily aimed at children aged 5 to 36 months.
Efficacy: Preliminary studies suggest it has an efficacy of around 77% over one year, which is notably higher than RTS,S.
Implementation: The vaccine has received regulatory approval in some African countries and is expected to play a significant role in malaria prevention efforts.