MALARVX'S VACCINE DEVELOPMENT
MalarVx is committed to the development of a safe, effective and affordable malaria vaccine. As a small and nimble company, we integrate cutting-edge technologies including repRNA with nanocarriers (LIONTM) proven superior safety, as well as genetically-attenuated whole parasites as vaccines to completely block the transmission of malaria in animal models.
We believe this integrated approach when tested in humans, will be instrumental in the long-term effort to eradicate both falciparum and vivax malaria in the world.
MALARVX'S PRIMING DOSE AS A REPLICON RNA VACCINE
Antigen-encoding repRNA
Antigen-encoding RNA has emerged as a valuable agent for vaccination. While mRNA has a short half-life and transient antigen production, self-amplifying (replicon) RNA (repRNA) initiates biosynthesis of antigen-encoding mRNA in the vaccinee, raising and prolonging antigen expression compared to mRNA with the same nanocarrier (Erasmus et al. 2020).
RepRNA also enhanced humoral (antibodies) and cellular immune responses when compared to conventional mRNA formulations, as shown by our collaborators HDT Bio, a Seattle-based biotechnology company, in immunogenicity studies on many emerging infectious diseases such as Dengue (Zhang et al. 2020), Zika (Erasmus et al. 2018), HIV (Khandhar et al. 2023), Mycobacterium avium (Rais et al. 2023), Crimean-Congo hemorrhagic fever virus (Leventhal et al. 2022), and SARS-CoV-2 (Erasmus et al. 2020; Kimura et al. 2023; Hawman et al. 2022).
repRNA-Prime and Trap vaccine
MalarVx developed a “prime-and-trap” (P&T) vaccine which combines a sub-unit (SU) of self-amplifying replicon RNA (repRNA) in a liquid nanocarrier as priming dose with a single “trapping” dose of cryopreserved radiation-attenuated sporozoites (RAS) (MacMillen et al. 2024), which providing better vaccine stability, improved logistics of storage and transport, and a more practical administration schedule.
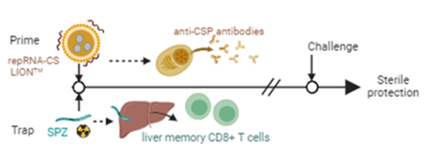
Using this repRNA technology, we developed a prime-and-trap (P&T) approach vaccine shown to engage both the humoral and cellular arms of the immune system against malaria infection (MacMillen et al. 2024). The subunit part of our vaccine consists of the repRNA encoding full length circumsporozoite surface protein (CSP) formulated with a novel Lipid InOrganic Nanocarrier (LIONTM) with enhanced stability, delivery, and immunogenicity, an innovative formulation developed by our collaborator at HDT Bio (Erasmus et al. 2020; Komori et al. 2023)
Innovative LION nanocarrier
LION nanocarriers formulation are highly immunogenic in malaria mouse models.
Our collaborators at HDT Bio have developed a new Lipid InOrganic Nanocarrier (LION) that was used to deliver repRNA to prime immune responses to the P. yoelii (Py) CS antigen (repRNA-PyCS).
Our repRNA-PyCS data support the robust and consistent immunogenicity of this platform and its ability to synergize with liver-targeted trapping vaccines to provide high levels of protection (MacMillen et al. 2024).
The use of LION nanocarriers accelerate the protective prime-and-trap vaccination schedule.
The accelerated schedule (same-day) of vaccine regimen induced >80% sterile protection compared to the standard 14-day schedule or the Trap alone dose showing <60% sterile protection following a challenge. (MacMillen et al. 2024)
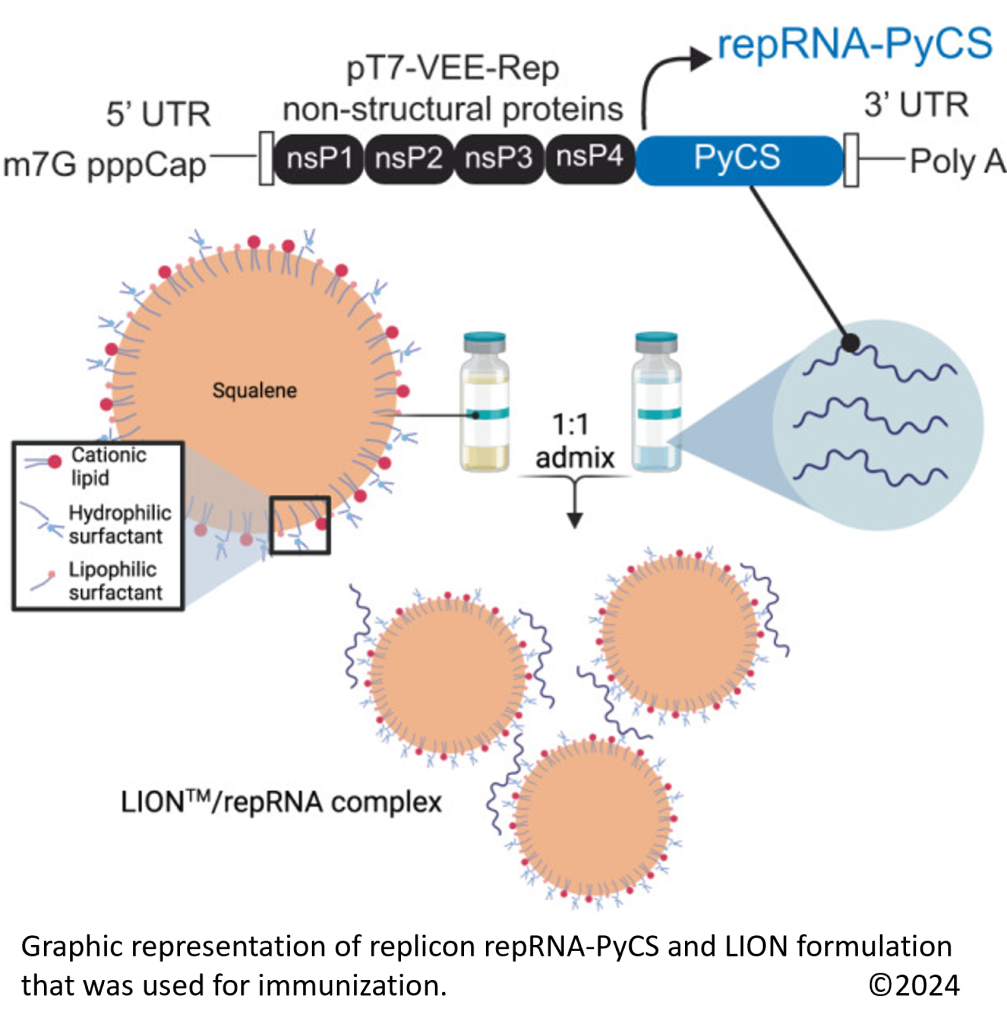
LION nanocarriers ease manufacturing of malaria vaccines.
Like for the GEMCOVAC™-19 vaccine based on repRNA-CoV2S (HDT-301: a single freeze-dried refrigerated vial and first approved for emergency use in India), the repRNA-LION formulation can by lyophilized and stable at 4oC for up to a year or at room temperature for several days, so it does not require ultra-cold storage, lowering the costs and facilitating distribution worldwide.
